 Airborne filamentous fungi (molds), including Aspergillus fumigatus and the Mucorales, are important human respiratory pathogens in an expanding population of patients with complex immunometabolic defects.
Airborne filamentous fungi (molds), including Aspergillus fumigatus and the Mucorales, are important human respiratory pathogens in an expanding population of patients with complex immunometabolic defects.
The surge in development of precision-medicine therapies for malignant and autoimmune diseases, and the emergence of viral sepsis syndromes, has resulted in an unprecedented increase in incidence of invasive mold infections (IMI).
IMI are associated with limited response to antifungal therapy, substantial mortality, and enormous economic burden. Understanding pathogenesis of IMI is an unmet need for design of better therapies and the primary focus of our research group.
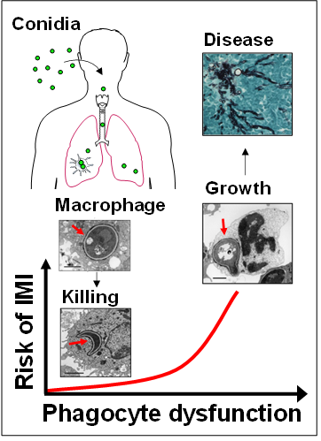
Dissecting the complex pathogenesis of IMI requires understanding of physiological antifungal host defense mechanisms. Protective immunity against molds is largely conferred by lung-resident macrophages and circulating phagocytes.
In healthy individuals, alveolar macrophages (AMs) phagocytose and eliminate hundreds to thousands of inhaled fungal conidia (spores) on a daily basis. The molecular mechanisms regulating intracellular fate of fungal conidia inside the phagolysosome of AMs remain largely unexplored. In immunocompromised patients, incompletely characterized immunometabolic defects in AMs result in intracellular fungal growth, tissue invaion and development of disease (cartoon).

